

Quantitative results are possible for plasma only.
This test can confirm Human Immunodeficiency Virus type 1 (HIV-1), infection by detecting HIV-1 RNA in both serum and plasma. Concentrations are reported in IU/mL for this assay.Ī "Not Detected" result indicates that HCV RNA was not detected in the patient’s serum or plasma.Ī result of 100,000,000 IU/mL indicates a current HCV infection and HCV RNA was detected but was above the Upper Limit of Quantification (ULoQ) and could not be quantified.Īn "Invalid" result occurs when an error occurred during testing. This test can confirm hepatitis C virus (HCV) infection by detecting and calculating the concentration of HCV RNA in serum and plasma.
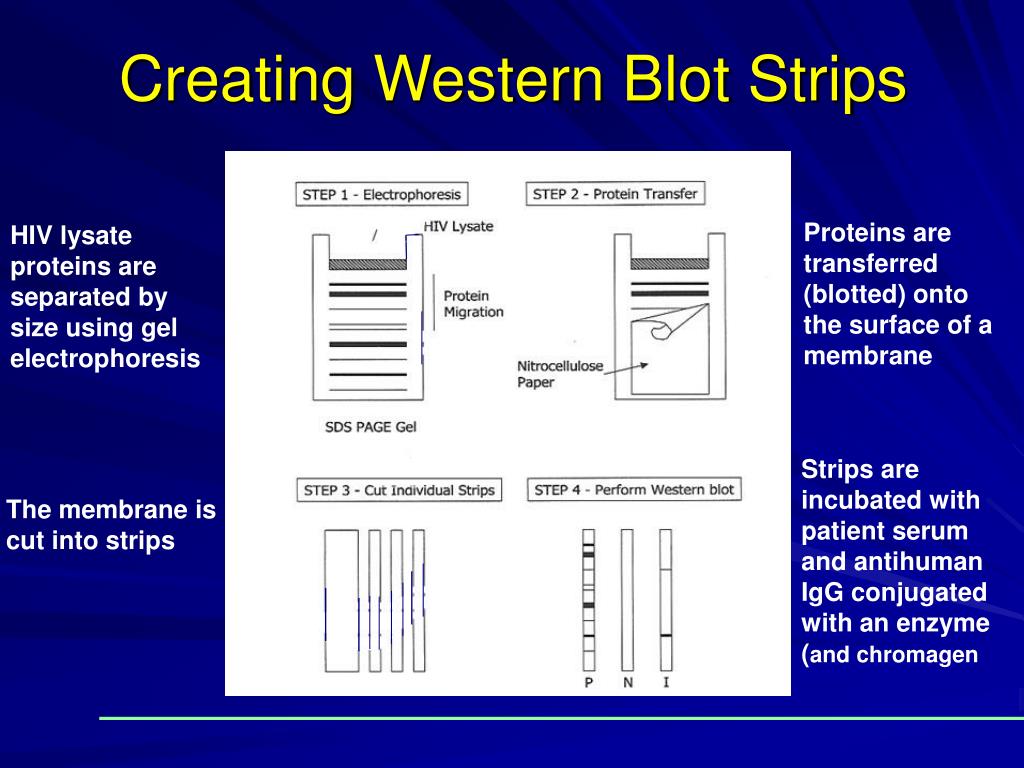
RNA Quantification by Real-Time Transcription Mediated Amplification. Sera positive for HCV by EIA method should be confirmed by additional supplementary test. Low reactive results are further tested for the presence of HepBcore antibodies. Repeatedly high reactive sera are not confirmed by a confirmatory test at TDSHS Laboratory. Antibody to HBsAg without anti-HBc develops in persons who receive hepatitis B vaccine. In the presence of antibody to HbsAg, a positive anti-HBc is a serological marker of past exposure.Īntibody to HBsAg with or without anti-HBc, specifies immunity against reinfection. In the absence of HBsAg and antibody to HBsAg, a positive anti-HBc is a serological marker of recent infection. The total antibody test to HAV is used to determine previous exposure to HAV and assess immune status.Ī positive test is indicative of recent infection with HAV. Measures the total antibodies to HAV (IgG, IgM and IgA) and is positive in acute hepatitis A and remains positive indefinitely. Serological cross-reactions with other flaviviruses (SLE and WN) preclude definitive diagnosis without confirmation by serum neutralization from the CDC.Ī 4 fold or greater rise in titer between acute and convalescent sera, collected four weeks apart, is evidence for infection with Ehrlichia.Ī 4 fold rise in IgG antibody titer or the presence of IgM in acute-phase sera is diagnostic for hantaviral disease. Reactive IgM result in a single serum sample is considered only presumptive evidence of recent infection, because the IgM can be detected for months and up to a year after Dengue infection. Additional testing is required to resolve the discordant results.Ī 4 fold change in antibody titer or the presence of Dengue-specific IgM in CSF confirms a recent infection. There is a high probability of non-infection.Īn equivocal result indicates that the presence or absence of antibodies to Trypanosoma cruzi could not be established with either assay.Īn inconclusive result indicates that results between the two assays did not agree. All specimens with presumptive positive, equivocal, and inconclusive results will be forwarded to the Centers for Disease Control and Prevention (CDC) for confirmatory testing.Ī presumptive positive result indicates that antibodies to Trypanosoma cruzi were detected for both assays.Ī negative result indicates that antibodies to Trypanosoma cruzi were not detected for both assays. Results from both assays are considered when determining the final result interpretation. The most convincing evidence of recent infection is a 4 fold rise in titer between an acute and convalescent serum.Īll specimens submitted for Chagas IgG serologic screening will be tested using two different enzyme immunoassays, both designed to detect antibodies to Trypanosoma cruzi, the causative agent of Chagas disease. You can contact commercial laboratories for Toxoplasma and Bartonella antibody testing, if needed.Ī single titer >= 1:160 is evidence of a prior infection, but does not confirm that it was recent. These sites may also not be accessible to people with disabilities.Įffective immediately, Toxoplasma and Bartonella IgG Antibody tests will no longer be offered at the DSHS Laboratory due to unavailability of reagents. Note: External links to other sites are intended to be informational and do not have the endorsement of the Texas Department of State Health Services. Health Care Information Collection (THCIC).National Electronic Disease Surveillance System (NEDSS).Emergency Medical Services (EMS) Licensure.Asbestos Hazard Emergency Response Act (AHERA).Food Manufacturers, Wholesalers, and Warehouses.Resources for Cancer Patients, Caregivers and Families.Cancer Resources for Health Professionals.Texas Comprehensive Cancer Control Program.Library and Information Science Program.Research, Funding, & Educational Resources.Center for Health Emergency Preparedness & Response.


 0 kommentar(er)
0 kommentar(er)
